We have updated our national guidance the NHS Fetal Anomaly Screening Programme (FASP) handbook and the FASP handbook for laboratories to help ensure local screening services meet national standards.
One of the main roles of Public Health England's (PHE) screening quality assurance service (SQAS) is to carry out formal visits to antenatal and newborn screening services. SQAS works with professional and clinical advisors when visiting laboratories providing screening tests for: …
The annual newborn blood spot screening data collection and performance analysis report for the year 1 April 2016 to 31 March 2017.
Ruth Stubbs, PHE's Cervical Screening Programme Manager, shares how the plans for introducing HPV primary screening are coming along.
The NHS Sickle Cell and Thalassaemia Screening Programme has organised 2 laboratory update days in April.
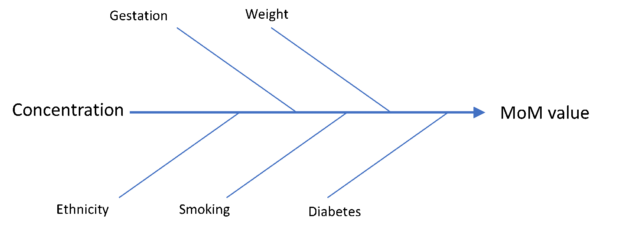
Shared learning in this blog post is specifically aimed at laboratories that provide Down’s, Edwards’ and Patau’s screening.
PHE Screening has published guidance for laboratories and local cervical screening providers to support the option of mitigating against the risk of longer cytology backlogs by using primary HPV screening pilot sites.
...hard work in this time of change. Information updates NHS England will provide further updates on the different stages in the procurement as we move further into the planning and...
...of an updated version of the antenatal laboratory handbook for the NHS Sickle Cell and Thalassaemia (SCT) Screening Programme. The updated antenatal laboratory handbook for the NHS Sickle Cell and...
...National Portfolio Lead for Cervical Screening. This means I have responsibility for representing cervical screening within the Screening Quality Assurance Service (SQAS). I thought it would be useful to explain...