Evidence shows that human papillomavirus (HPV) testing is a better way of identifying women at risk of cervical cancer than the cytology (smear) test that examines cells under a microscope.
Last year, after reviewing the evidence, the UK NSC recommended that the HPV test should replace cytology as the first stage in cervical screening.
This will be a major change for the NHS Cervical Screening Programme (NHSCSP). An HPV primary screening implementation group of PHE Screening and NHS England experts is developing plans for how to roll out HPV primary screening nationally in England.
On 10 January 2017 the implementation group hosted an options appraisal meeting to consider the possible models for implementing HPV primary screening across England.
The Royal College of Pathologists, the British Association for Cytopathology and the Institute of Biomedical Science supported and informed this group. Dr Karin Denton, Regional Head of the Screening Quality Assurance Service, led the options appraisal meeting and it was facilitated by Costello Consulting. Many thanks to everyone for their time and support with this piece of work.
The options appraisal considered 10 important objectives for each model (see diagram below).
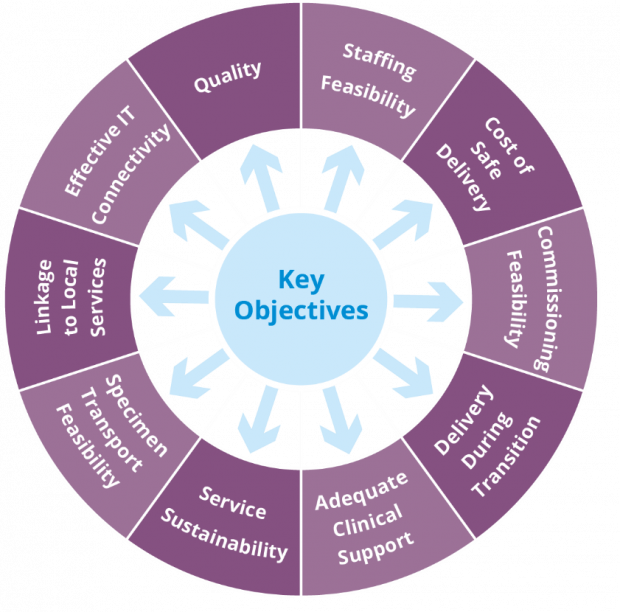
The options appraisal group previously met before Christmas to discuss and agree the relative importance of each of these 10 areas with stakeholders and agree how they should be weighted. The results of this discussion can be seen in the table below.
Quality and safety was weighted most important, reflecting the aim of screening to prevent as many cervical cancers as possible and maintain the highest standards.
Staffing capacity and maintaining a resilient service were also weighted highly along with effective IT connectivity and cost of safe delivery.
| Objective | Weight |
| Quality | 18 |
| Staffing feasibility | 15 |
| Effective IT connectivity | 15 |
| Cost of safe delivery | 15 |
| Linkage to local services | 10 |
| Delivery during transition | 6 |
| Adequate clinical support | 6 |
| Service sustainability | 6 |
| Specimen transport feasibility | 5 |
| Commissioning feasibility | 4 |
| Total | 100 |
The options appraisal group considered 6 options in total.
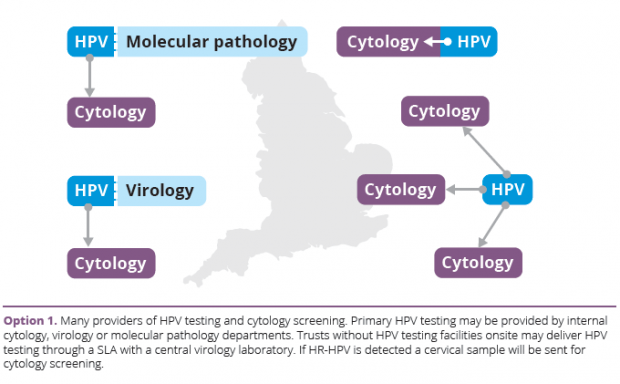
- Many providers delivering HPV testing and cytology testing (aligns with current cervical screening model).
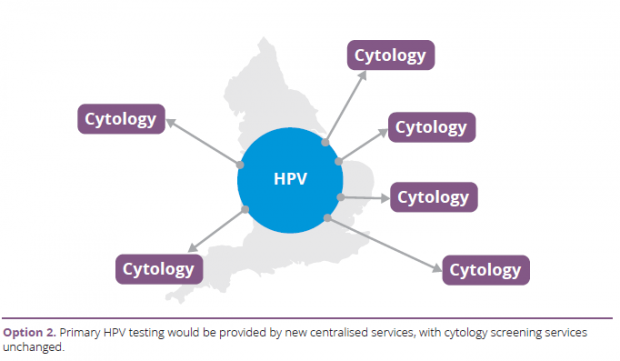
- Centralisation of HPV testing with cytology testing from existing providers.
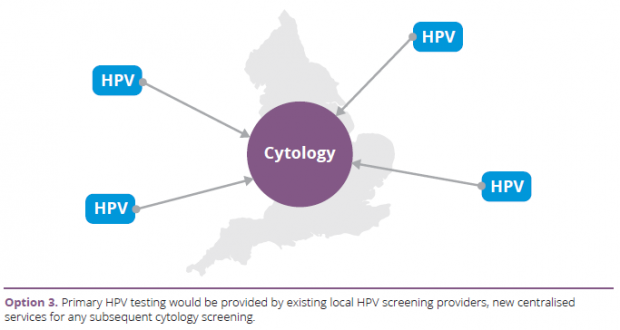
- Centralisation of cytology testing with HPV testing by many, mainly local, providers.
- Centralisation of HPV testing and cytology testing as a single seamless service, using:
- a minimum number of 4 to 5 centralised services performing both HPV testing and cytology screening (option 4a)
- a maximum number of 10 to 15 centralised services performing both HPV testing and cytology screening (option 4b)
- A network approach of many providers of HPV and cytology screening.
Recommendations
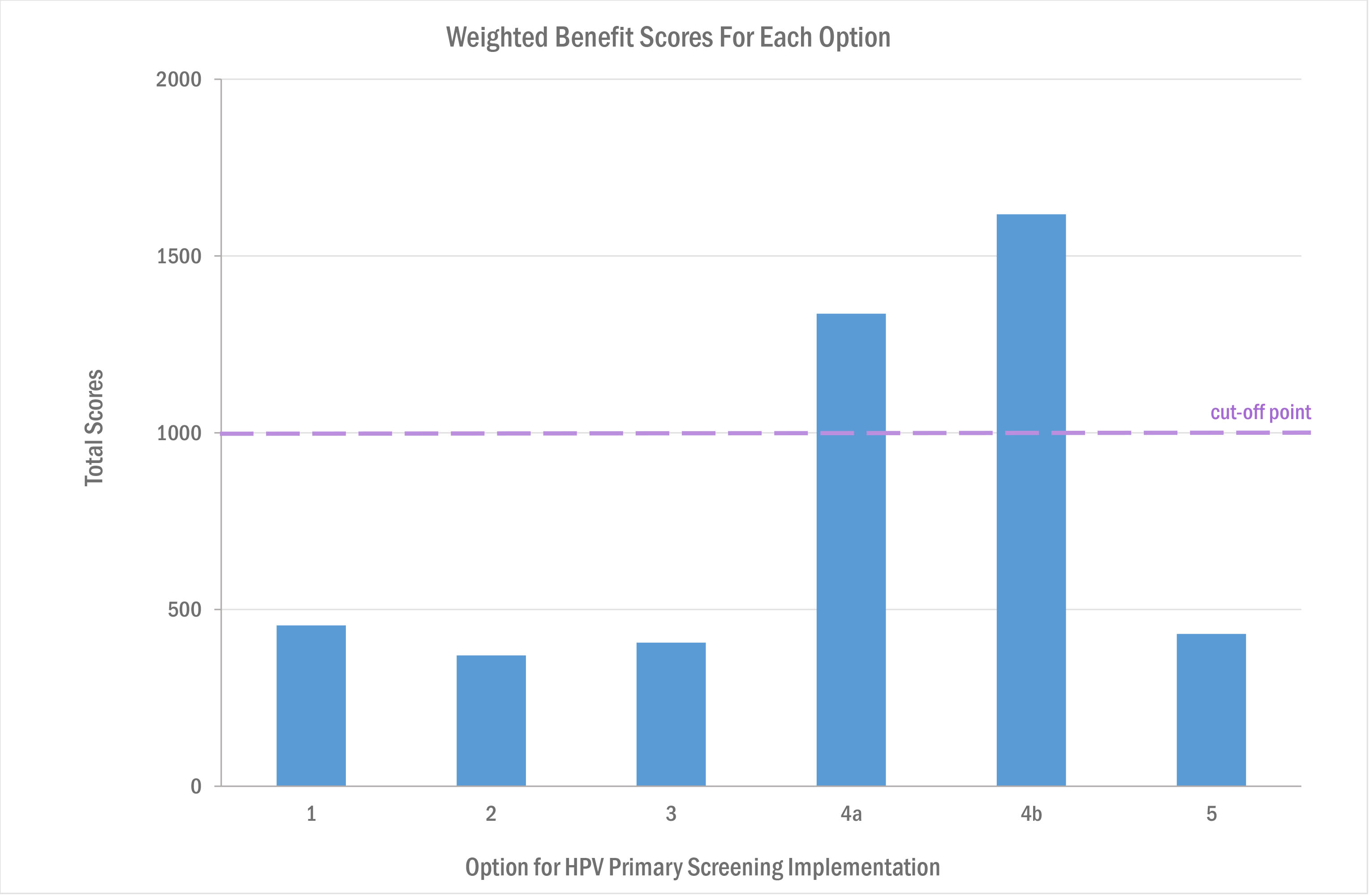
After evaluating and scoring each of the proposed option models, the options appraisal group identified centralisation of HPV testing and cytology testing as a single, seamless service as the recommended model of delivery.
Options 4b (using a maximum number of 10 to 15 centralised services) and option 4a (using a minimum of 4 to 5 centralised services) both scored highly in terms of quality and safety. These were the only options to score ‘above the line’ for acceptability, achieving a total weighted score of more than 1,000 points (50% of the maximum score possible). See table below.
Next steps
NHS England, as commissioners of cervical screening, will now take forward work to make sure that the preferred options are deliverable.
This work will include assessing costs, procurement arrangements, due diligence, the implication of each option on existing services and appropriate sign-off processes. Timescales for this work will be shared once available.
Janet’s new role
I’m pleased to be able to announce that Janet Rimmer has been appointed senior implementation lead for HPV primary screening.
Janet has extensive experience of working in cervical screening and will work with the NHSCSP team to take forward this work.
PHE Screening blogs
PHE Screening BLOGs provide up to date news from all NHS screening programmes – replacing our previously published newsletters.
You can register to receive updates direct to your inbox, so there’s no need to keep checking for new blogs.
34 comments
Comment by Cytologist posted on
Is there any further update, including news of when commissioning and procurement of the Primary HPV service can start? We are now another month on from the last comment posted, and edging ever closer to the December 2019 deadline with no obvious progress.
Comment by Mike Harris posted on
Thank you for your comment. PHE provided a recent update to the British Association for Cytopathology and this is now featured on their website. There is an HPV Implementation Board meeting later this month when it is anticipated that NHS England will provide an update on the commissioning and procurement arrangements for HPV primary screening implementation. When this information is available, NHS England will provide an update and PHE will provide a further update to the blog. Thank you for your patience.
Comment by Ruth Stubbs posted on
Thank you for your comment. PHE provided a recent update to the British Association for Cytopathology and this is now featured on its website. There is an HPV Implementation Board meeting later this month and it is anticipated that NHS England will provide an update on the commissioning and procurement arrangements for HPV Primary Screening Implementation. When this information is available, NHS England will provide an update and PHE will provide a further update via this blog. Thank you for your patience.
Comment by Amanda posted on
With reference to the primary HPV screening pilot sites (Sheffield, Cumbria etc) what percentage of colposcopy patients at these sites are now being referred under the primary HPV screening algorithm?
Is primary HPV screening now the standard of care at these sites? if not, when would you expect them to covert fully to the new pathway?
When do you expect Primary HPV screening to be fully rolled out across England?
Comment by Ruth Stubbs posted on
This varies by pilot site. Initially in 2013 all six pilot sites converted a minority proportion of their cytology workload to primary HPV testing (20 to 45%) for the purposes of the pilot. In recent months however three of the pilot sites have also begun primary HPV testing a larger proportion of their screening workload in order to free up cytology capacity to help with cytology backlogs. Therefore, in some pilot sites the proportion of women referred to colposcopy under the primary HPV testing pathway is now greater than half of the laboratories workload, whilst the remainder are still referred via the primary cytology and HPV triage pathway.
Further to this colposcopy referrals are greater under the primary HPV testing pathway compared to cytology screening in the second and third years of implementation.
Primary HPV testing will be fully implemented throughout England by the end of December 2019.
Comment by Nick posted on
My understanding was that in January this year the rollout was supposed to by by April 2019 now 7 months later it is has been delayed 8 months till December 2019 not much progress!
Also I assume there are many more replies to this blog than the limited number that are published here, why are not all replies available to be seen?
Comment by Ruth Stubbs posted on
Thank you for your comment.
Achieving world-class cancer outcomes: a strategy for England 2015-2020 (https://www.england.nhs.uk/cancer/strategy/) recommends that HPV Primary Screening will have full national coverage by 2020 and PHE with NHS England are working to meet this recommendation.
We publish the vast majority of comments posted on the PHE Screening blog in line with our comment and moderation guidelines (https://phescreening.blog.gov.uk/terms/).
Kind regards
Comment by Biomedical Scientist- Cytology posted on
When HPV Primary Screening is approved and the hospitals that are going to perform HPV primary screening are confirmed. How will staffing be handled? Do people get to carry on working in Cytology purely because of the fact that they are already at the hospital that will be a site for HPV primary screening? Or will there be fairness in that people who want to continue working in Cytology, from other hospitals, will get to be interviewed and then the best candidates will be chosen to work and start HPV primary screening?
I think it is unfair that there are fresh new talent in Cytology that are looking forward to working in Cytology and their future has been cut short purely because they are not staff at the hospital that will be the site for HPV primary screening.
Also will the hospitals that are doing HPV primary screening need more staff?
Comment by Ruth Stubbs posted on
Thank you for your comments. Staffing of HPV primary services and contractual issues around transfer are within the responsibility of NHS England. NHS England are currently focused on engagement with local commissioning teams in relation to the development of the commissioning and procurement strategies. Further work to identify and understand the current position regarding laboratory configuration and networks, local pathology strategies, the existing contractual frameworks is being undertaken.
Ruth Stubbs
National cervical screening programme manager
Comment by Cytologist posted on
Is there a time frame for the next update on HPV primary screening as it has been 2 months since this blog was published?
Comment by Ruth Stubbs posted on
Thank you for your question.
Following the recommendations made by the options appraisal stakeholder group on 10 January, NHS England was required to undertake a due diligence exercise to ensure the recommended options for laboratory centralisation were viable, affordable and operationally deliverable. Although this process is well under way, it is important it is undertaken thoroughly and NHS England will be informing the HPV Implementation Group of the outcome as soon as it is complete. The outcome of this process will be also communicated to stakeholders via the national PHE blog and the local public health commissioning teams.
Regarding the process to commission and implement the delivery of the HPV primary screening test, as part of the national cervical screening programme, this will require a procurement process in order to comply with EU regulations. The procurement will be led by NHS England as the accountable organisation for the delivery of the public health section 7a cervical screening programme. Further information about the procurement process will be made available from NHS England, following assessment of the recommendations from the HPV Implementation Group about laboratory footprint.
We hugely appreciate your patience and support with this process, and will keep you updated on any important developments over the coming months.
Comment by Cytologist posted on
Is there any update available regarding HPV primary screening? We have not been updated since January.
Comment by Ruth Stubbs posted on
Thank you for your comment. NHS England has provided the following update:
Following clinical recommendations regarding the configuration of laboratories to support the changes to the NHS Cervical Screening Programme made by the Options Appraisal Stakeholder Group, NHS England has been undertaking a series of appraisals to determine the affordability and operational deliverability of the options. Alongside this, the broader impact of any strategic pathology reforms and impact that may have on laboratory configuration for delivery of the cervical screening pathway must be considered. Therefore, NHS England intends to carry out a series of engagements with its local commissioning teams and Public Health England to ensure that any decisions on the number of laboratories and their footprint does not contradict existing strategies.
We recognise that this process is taking longer than anticipated, and hope you will appreciate that it is of upmost importance that the right decisions on these service changes are made. NHS England will be informing all stakeholders of this outcome as soon as this process is complete.
Regarding the process to commission and implement the delivery of the HPV test as the primary screening within the NHS Cervical Screening Programme, the central public health team of NHS England will be working closely with local commissioning teams to explore available options in relation to securing contracts to deliver this change in service. This may require a procurement process in order to comply with EU regulations. If so, this will be led by NHS England as the accountable organisation for the delivery of the Public Health Section 7a Agreement.
We hugely appreciate your patience and support with this process, and will keep you updated on key developments throughout the next couple of months.
Comment by Duncan posted on
Not all HPV or CIN1/11/111 will progress to cervical cancer, in fact most will not. Therefore is it fair that each year nearly 200,000 women are sent off to colposcopy for further testing and likely treatment for something they do not actually have or will ever get? Collateral damage springs to mind. These are invasive tests and treatments that can have consequences to a woman's future health. After all cervical cancer is rare and always was rare. Would it not be better that the huge cost of this programme be better spent elsewhere.....on symptomatic patients? Nearly every woman I know thinks they have been saved from cc because they had treatment/s when really it is not known which abnormal cell will change and which will not, bearing in mind most will not. Abnormal cells can be from Hormones(menopause)/toiletries/sex and many other reasons. With HPV the woman is treated then she returns back to her partner who most likely has HPV, merry-go-round for little benefit. Also if HPV is so common in sexually active people will this not increase the colposcopy visits unnecessarily then increasing overtreatments. A bit like looking for a needle in a haystack - CC risk of the average woman is around 0.65%, that's very small but the false positive rate is very high. So 99.35% no risk of cc, seems good odds to me. All screening has associated risks/overtreatment. Shouldn't the money be spent on patients who are really ill/unwell? Instead of all the cutbacks NHS is making?
Comment by Ruth Stubbs posted on
Thank you for sharing your views. Tthe programme is currently undergoing a change to HPV as a primary screening test using cytology as a triage. This is a significant change to the programme. HPV is a very common infection. About 80% of women will have an HPV infection over their life time but very few will go on to have cervical cancer. Therefore, it is said the HPV is a necessary cause for cervical cancer but not sufficient to cause it. While smears tell us of current abnormalities in the cervix HPV tells us about future risk of cervical cancer. Both tests together allow us to identify women who are at particularly high risk of the disease. This enables a reduction in the number of women referred to colposcopy for further investigation. This provides not only a more personalised assessment of risk but also better value for money. Additionally, by minimising the number of women referred for further investigation, we limit harms associated with screening. We hope you will appreciate that the cervical screening programme continues to develop and improve as new evidence emerges.
Comment by Liz Lewis posted on
Is it absolute that all cervical changes are from HPV, my understanding that it is high percentage but not 100% if so what of those few who are HPV clear.
Comment by Ruth Stubbs posted on
Thank you for your comments and question. Evidence shows that 99.7% of cervical cancers contain high risk (HR) HPV DNA. Looking at cases of cervical intraepithelial neoplasia (CIN), the higher the grade of CIN, the more frequently HR-HPV is found. This suggests that women who do not have HR-HPV are extremely unlikely to develop cervical cancer in the short to medium term.
Comment by katrehman posted on
I don't work in health care but I did get made redundant 3 years ago when my employer went bust so understand its a horrible thing to happen however here we are talking about health care and the move to HPV testing which is supposed to benefit women. There fore surely that's the most important factor..to improve womens health! I'm personally not a fan of the cervical screening programme and also feel if less money was wasted on campaigns designed to scare us into screening. .if less was spent chasing women who choose not to screen...there might be more money available to fund new jobs elsewhere and also fund the self testing option the Dutch have had since the beginning of last year. Surely you MUST see that women aren't being swayed by these scare campaigns and LOATHE this outdated test??
Comment by Ruth Stubbs posted on
Thank you for sharing your opinions. The NHS Cervical Screening Programme is currently considering self-testing options which will become a possibility once HPV primary screening has been implemented.
Comment by Concerned Cytologist posted on
Will NHS England consider the current location of essential staff required to deliver the reconfigured service to ensure the location of the new provider laboratories are within reasonable distances? Previous experience has shown that if this is not considered, the staff required to deliver the service will not transfer and the new provider will be unable to deliver due to insufficient staff. This is particularly relevant for Option 4a.
Comment by Ruth Stubbs posted on
Thank you for your comment.
As this question relates to NHS England, PHE is not in a position to comment. NHS England is currently considering the next steps from the options appraisal recommendations. These include:
• working closely with the national procurement team to understand what procurement arrangements would need to be put in place for each option and the timescales for this
• liaising with the local commissioning teams to understand the cost implications if either option was to be implemented and to undertake financial modelling
• working with other national teams (such as primary care services) to understand the impact that either option would have on current service delivery
• ensuring that both options have been considered in a wider context, including the impact on the s7a agreement and also the sustainability and transformation plan agenda
Comment by Callum posted on
Thank you for the blog which gives some clarity on laboratory configuration.
As Hospital Based Programme Coordinator I have responsibility across cytology, colposcopy and histology and regardless of cytology configuration need to plan delivery of the colposcopy and histology service.
You must by now have data from the pilot sites covering the first three years of HPV primary screening. Please could you tell me, for each of the first three years, the percentage increase or decrease in colposcopy referrals and biopsy/LLETZ specimens.
Alternatively if this information is in the public domain , where can I find it?
Comment by Ruth Stubbs posted on
Thank you for your comment on the blog post. I am pleased you found it helpful. The data from the HPV pilot sites is still in draft and has not yet been published. We have asked for the timescales for data publication and hope that it is not too far away.
Comment by worried screener posted on
Does NHS England have a redundancy package to help pay for the inevitable job losses if option 4a is selected?
Comment by Ruth Stubbs posted on
Thank you for your comment. Unfortunately Public Health England cannot advise on this aspect of the HPV Primary Screening implementation as it is in the remit of NHS England. NHS England will consider the impact of the recommendations from the options appraisal on the sustainability and transformation plans.
Comment by Karen Snelgrove posted on
How do you envisage colposcopy MDT meetings to factor in these proposed arrangements?
Comment by Ruth Stubbs posted on
Thank you for your comments. The NHS Cervical Screening Programme standards and guidance are being reviewed and updated to ensure they will meet the requirements of HPV primary screening. Detail of the MDTs will be included in programme guidance and the service specification for the cervical screening. It is agreed that MDTs will need to continue to be run in a way which is accessible to colposcopy, cytology and histology but the way the meetings are delivered and which staff attend may change.
Comment by Irene Stratton posted on
It's an exciting opportunity for education about the risk factors for cervical cancer. I worked in the unit where much of the work was done in the 1980s about the role of viruses and the increase in risk with increasing number of sexual partners and in those who had sexual relationships at younger ages. When I first started work there it was thought that cervical cancer might be linked to smoking and sunbathing! By the time women enter the screening programme of course it may be too late for them to consider a change in behaviour or use of barrier contraception but they might be able to talk to their daughters, sisters and younger friends.
So will PHE be explaining why the change in screening method is happening?
Comment by Ruth Stubbs posted on
Thank you for your comments, PHE provides detailed leaflets for women who are invited for screening and specific leaflets and information sheets for those who are invited for HPV Primary testing. This provides information on HPV and why the screening method has changed. Further information is available on <a href="http://www.nhs.uk/Conditions/Cervical-screening-test/Pages/Introduction.aspx">NHS Choices</a>.
The <a href="http://www.nhs.uk/conditions/vaccinations/pages/hpv-human-papillomavirus-vaccine.aspx">NHS childhood vaccination programme</a> offers the HPV Vaccination to all school girls age 12 to 13 years. This provides a further opportunity to educate on the HPV virus and the role it plays in cervical cancer.
Comment by Deirdre posted on
Public ignorance about the HPV virus, and the limitations of the smear test is maintained to keep women blindly following the programme. We see campaign after campaign by misguided individuals believing the smear to be a diagnostic test and even a cure for cancer. If a test is promoted to save a life at 25, but not be available for for a 24 year old, it is bound to get individuals setting off these campaigns, and the tabloids and charities are only too pleased to spot the financial gains that can be made from fanning the flames of misinformation.
The public must be given the honest accurate facts about smear tests-when they work and when they don't work, and what they can and cannot do.
Comment by Ruth Stubbs posted on
Thank you for sharing your views. The aim of the cervical screening programme is to reduce the number of women who develop invasive cervical cancer and the number of women who die from it. We do this by inviting women for screening aged 25 to 49 every 3 years and women aged 50 to 64 every 5 years. The programme looks to prevent cancer by detecting abnormal cells that could, if left undetected, turn into cancer. The programme provides <a href="https://www.gov.uk/government/publications/cervical-screening-description-in-brief">information leaflets</a> in each invitation letter to provide women with information to help to make their choice on whether to accept the screening invitation. The programme does not advocate that the screening test is a diagnostic test. The decision to offer screening from age 25 was based on evidence (Sasieni et al BMJ 2003) which suggested that screening under age 25 was not effective at preventing cervical cancer. This evidence has been reviewed many times over the years and has continued to receive support from the organisation charged with reviewing evidence and issuing recommendations for screening in the United Kingdom, the <a href="https://legacyscreening.phe.org.uk/cervicalcancer">UK National Screening Committee</a>.
Comment by Phil Bullock posted on
PS Its also rather surprising to see that a history of robust service delivery does not feature as a parameter for assessment.
Comment by Ruth Stubbs posted on
Dear Mr Bullock
Thank you for your comment. Please note the objectives related to ‘cost of safe delivery’ not to ‘costs’ alone. Estimates of comparative costings of the different service configuration options were considered. We did not consider any issues of test platform selection as this does not impact on service configuration. We are pleased to assure you that these objectives were fully informed and supported by clinicians representing the professional bodies mentioned in the blog.
Kind regards
Ruth Stubbs
Comment by Phil Bullock posted on
These factors on the face of it look to be those of greatest significance and weighted appropriately. But can I ask please...when costs are considered is that just the cost of laboratory testing or does it include sample taking and colposcopy referral costs? There are likely to be significant variances in these costs dependant on the chosen HPV testing technology - a cheaper HPV test may not be the most cost effective for the programme if a higher number of repeat tests and/or colposcopy referrals follow.