We’ve spent the last couple of months reviewing the standards for newborn blood spot (NBS) screening and would like to invite feedback from all our stakeholders.
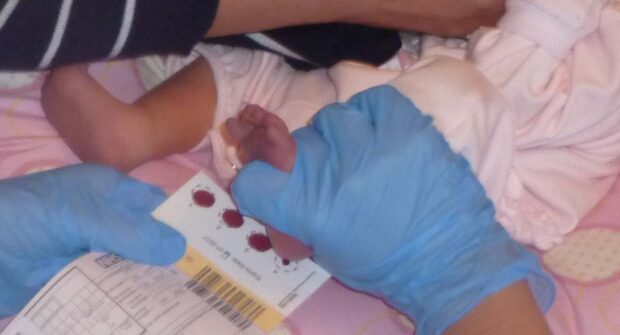
The NBS programme has used standards for over 10 years to help monitor the effectiveness of the screening pathway. It’s a complex programme that involves different organisations working together.
The standards are a set of targets that aim to support providers and commissioners provide a safe and effective screening service. They also help to drive improvements.
We last updated the NBS standards in 2013. We’ve asked clinical, laboratory and quality assurance colleagues to help us revise them to reflect recent changes to the programme, including the addition of 4 metabolic conditions. We’re now asking for help from all our stakeholders to make sure they are fit for purpose.
How to respond
- Please open and review the draft revised standards on the GOV.UK consultation page.
- Please then click on the SelectSurvey link on the page and complete the consultation survey. The consultation will be open for 4 weeks until 26 September 2016.
Please share this blog with people you work with. We’re interested in everyone’s thoughts.
You can access the current NBS standards for reference.
Proposed changes include:
- updates to some thresholds to reflect recent performance
- report PKU coverage (standards 1a and 1b) as proxy for all 6 inherited metabolic diseases
- focus on barcoded NHS number labels in standard 3 to drive increased use
- focus on day 5 only in standard 4 to improve timeliness of sample collection
- focus on day 21 for CF repeats in standard 7 to maximise accuracy of test and timeliness of referral
- remove laboratory accreditation standards (8 and 10) as they are in the service specification (Section 7a)
- develop an audit tool to replace standard 12 (timeliness of results to parents)
- if retain standard 12, introduce standard 12b for movers in
Reminder
You can also respond to the consultation on the revised sickle cell and thalassaemia screening programme standards until 9 September 2016.
What happens next?
We'll publish a summary of the consultation responses and get in touch with everyone when the final version is ready.
PHE Screening blogs
PHE Screening BLOGs provide up to date news from all NHS screening programmes – replacing our previously published newsletters.
You can register to receive updates direct to your inbox, so there’s no need to keep checking for new blogs.